The group of researchers at IIT-Madras behind the research
Researchers from the Department of Physics at IIT-Madras have developed vital elements for a extremely environment friendly, cost-effective solution to electrolyze seawater to generate hydrogen. The outcomes had been printed within the journal ACS Applied Energy Materials.
State-of-the-art alkaline water electrolyser expertise is energy-intensive, requires an costly oxide-polymer separator, and makes use of contemporary water for electrolysis. The IIT-Madras group led by Dr. Ramaprabhu Sundara has addressed every of those challenges by growing easy, scalable and cost-effective options which might be extremely environment friendly in splitting seawater and producing hydrogen.
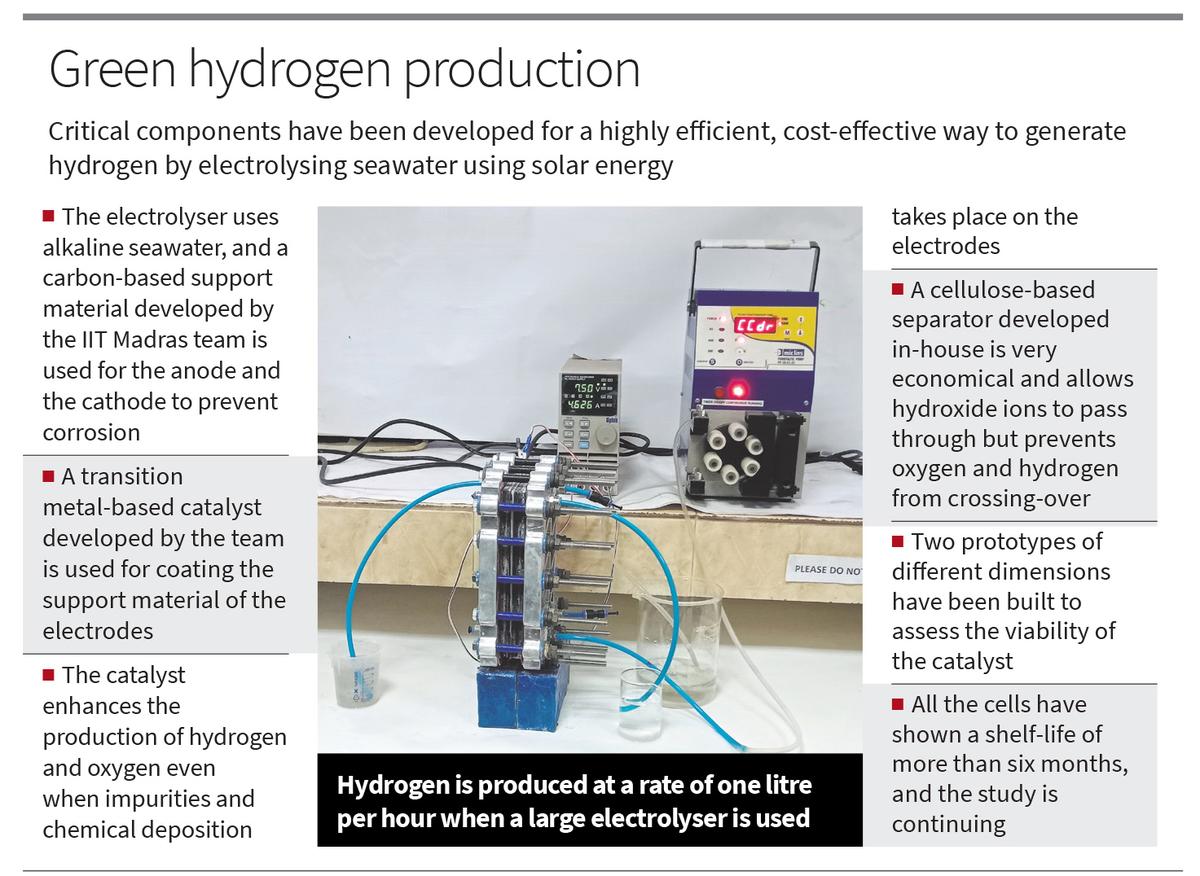
In place of pure or contemporary water, the group has developed an electrolyser using alkaline seawater. They used a carbon-based assist materials for the electrodes as an alternative of metals to nearly remove the potential of corrosion. They additionally designed and developed transition metal-based catalysts that may catalyse each oxygen and hydrogen evolution reactions. The catalyst enhances the manufacturing of each hydrogen and oxygen even when impurities and chemical deposition on one of many electrodes takes place. Also, the researchers have developed a cellulose-based separator that may be very economical and serves the aim of permitting hydroxide ions to go by way of however prevents oxygen and hydrogen which might be generated from crossing-over. Finally, the researchers have optimised all of the parameters such that the water electrolyser can immediately use photovoltaic-derived voltage to separate seawater and generate inexperienced hydrogen and oxygen, oxygen can be utilized elsewhere.
The reactions
Alkaline water electrolyser consists of two half-reactions occurring on the anode and cathode. At the cathode, water dissociates into H+ and hydroxide ions, and the H+ ions get transformed into hydrogen. The hydroxide ions produced on the cathode permeate by way of the separator and oxygen is generated on the anode.
When seawater is used for electrolysis, hypochlorite formation happens on the anode. Hypochlorite is accountable for corrosion of the electrode assist materials, and competes with the oxygen evolution response thus lowering the quantity of oxygen produced. At the cathode, the hydrogen evolution response is slowed down when a number of impurities get adsorbed on the electrode floor.
The electrodes have a assist materials that’s coated with a catalyst. “Since conventional metal support materials get easily corroded when seawater is used, we developed a carbon-based support material,” says Prof. Sundara. “The support material is used in both the anode and cathode, and is coated with the catalyst. The catalyst allows enhanced and simultaneous production of hydrogen at the cathode and oxygen at the anode.”
According to Prof. Sundara, the transition bimetals current within the catalyst are extra selective in direction of oxygen evolution response than hypochlorite formation. Thus the problem of hypochlorite formation lowering oxygen manufacturing is taken care of. Similarly, even whereas the cathode continues to adsorb impurities, the catalyst promotes the hydrogen evolution response, which helps within the elevated manufacturing of hydrogen.
The separator
Another distinctive function is the novel separator that has been developed by the group. When alkaline electrolyte is used, the anode and cathode are separated with a separator. Since zirconium oxide-based materials that’s routinely used is pricey, they got here up with a cellulose-based separator which permits the hydroxide ions to go by way of from the cathode to the anode. But it minimises the crossover of hydrogen and oxygen that’s generated.
“We found our separator is highly resistant to seawater degradation,” says Sana Fathima, one of many co-authors of the paper.
“Using the assembled electrolyser, we have demonstrated an overall seawater splitting voltage of 1.73 V at 10 mA/sq.cm (a benchmark current density corresponding to about 12% efficient solar-to-fuel conversion device under 1 sun illumination) at 26 degrees C,” says Anamika Ghosh from IIT Madras and the primary creator of the paper. “We have optimised all the parameters such that the water electrolyser can directly use photovoltaic-derived voltage and work at 10mA/sq.cm current density to split seawater for green hydrogen production.”
The group has developed two prototypes of various dimensions to evaluate the viability of the catalysts. “In the case of the smaller electrolyser (16 sq.cm dimension) hydrogen is produced at a rate of 250 ml per hour, while in the larger one (391 sq.cm dimension) hydrogen is produced at a rate of about one litre per hour at an applied voltage of 2 V,” Ms. Ghosh says. “We also fabricated a stack consisting of three such cells and hydrogen produced is about four litres per hour at an applied voltage of 2 V per cell.” All the measurements had been achieved at ambient strain and room temperature.
“All the cells have shown a shelf-life of more than six months, and the study is continuing,” says Ms. Fathima.


