Photo used for illustration goal solely.
Much like many different viruses which have developed strategies to evade T cell-mediated clearance by people, SARS-CoV-2 virus too has the flexibility to evade the CD8 T cells. While neutralising antibodies are liable for stopping an infection, CD8 T cells play an enormous position in decreasing the viral load and clearing the an infection by detecting and killing contaminated cells. The CD8 T cells can not stop an infection.
A research just lately printed within the Proceedings of the National Academy of Sciences (PNAS) discovered that the SARS-CoV-2 virus encodes a number of viral elements that modulate main histocompatibility advanced class I (MHC I) expression within the host cells. The MHC I performs an essential position in alerting the immune system to virally contaminated cells. The MHC I molecules are expressed on the floor of all contaminated cells.
MHC I molecules
“When a virus infects a cell, one of the ways in which the immune system responds is by attaching short sequences of proteins from the virus (antigen) to MHC I molecules, thus presenting the antigen on the outside of the cell. Killer T-cells look for antigens inside MHC I and if they find any that match the specific thing they are programmed to kill, they go ahead and kill it.” One of the widespread tips that viruses use to keep away from killing is to inhibit MHC I expression and presentation. SARS-CoV-2 isn’t any exception. The SARS-CoV-2 virus has developed a number of strategies to inhibit MHC I expression, which isn’t seen within the case of the influenza virus. The suppression of MHC I is particularly seen within the contaminated cells and varies between completely different viral strains.
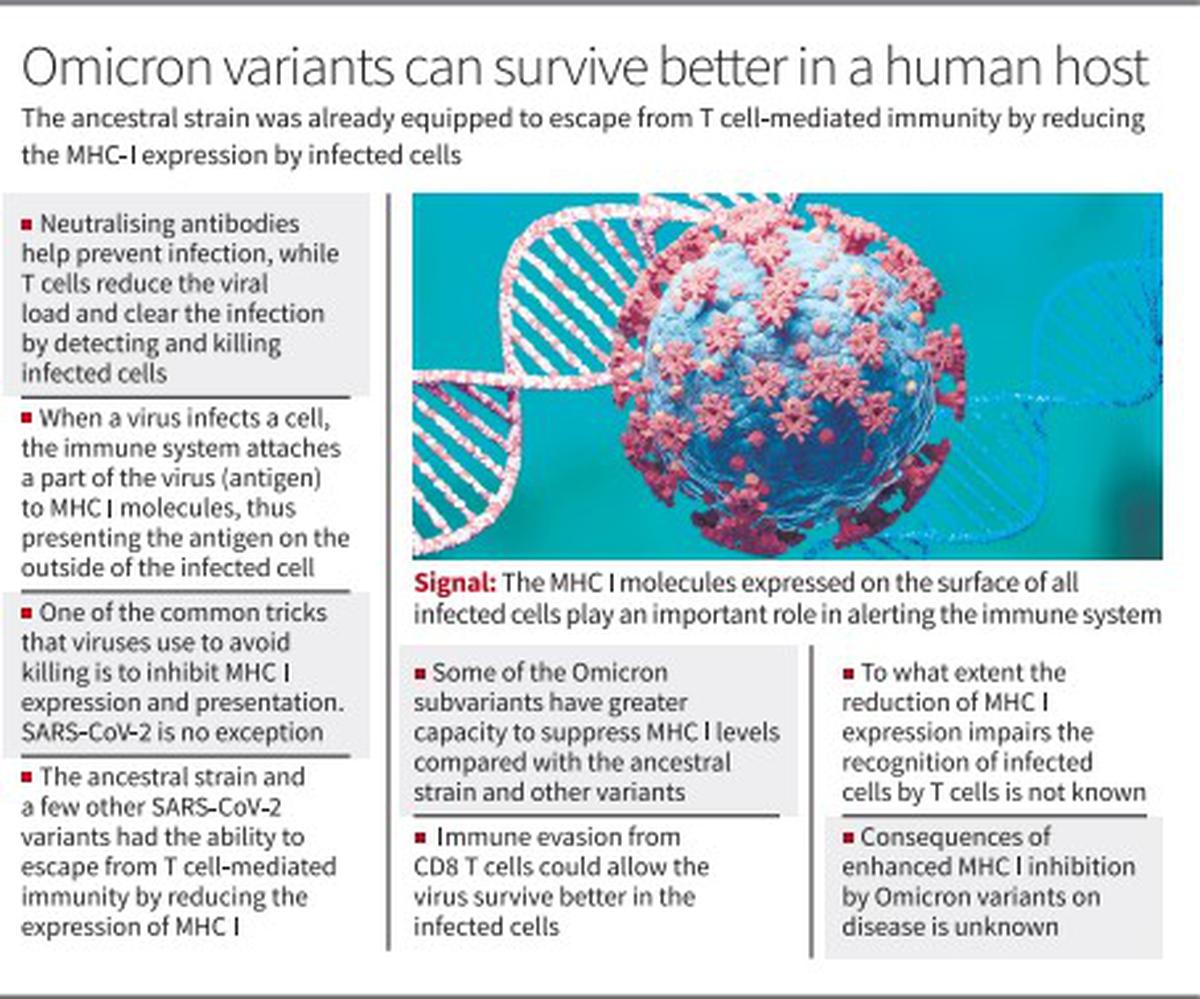
“Our data showed that MHC I suppression is mediated by a number of viral gene products and affects only the infected cells. Such a mechanism will not lead to generalised immunodeficiency but reflects a specific survival mechanism for SARS-CoV-2,” Dr. Akiko Iwasaki, Yale University immunologist and the corresponding writer of the paper, tweeted.
“What does this mean? Immune evasion from CD8 T cells could allow the virus in infected cells to survive better in the host. The virus could establish a safe niche for prolonged replication. To eliminate such persistent reservoirs, we need to employ antivirals or antibody therapy,” she stated in one other tweet.
‘Superior capacity’
The ancestral pressure first present in Wuhan, China and some different variants that got here up later already had the flexibility to escape from T cell-mediated immunity by decreasing the expression of MHC I. But the authors discovered that the Omicron subvariants (BA.1, BA.2.12.1, XAF, and BA.4) had a “superior capacity” to suppress MHC I ranges on the floor of the cells contaminated by the virus in contrast with the ancestral pressure and different variants. Besides being endowed with larger means to evade neutralising antibodies, the Omicron subvariants are higher at evading recognition by the killer T cells.
Looking for the molecular mechanism of the improved MHC I inhibition by Omicron subvariants, the crew, led by Dr. Iwasaki, recognized widespread mutations within the E protein (T9I) that are shared amongst all Omicron subvariants used within the research.
“We found that T9I mutation within the E protein significantly enhanced the degree of MHC I downregulation. The results underscore the universal capacity of all SARS-CoV-2 strains to mediate the cell-intrinsic reduction of MHC I expression within the infected cells, and highlight the superior ability of the Omicron subvariants in acquiring MHC I evasion capacity,” they write.
Mice contaminated with SARS-CoV-2 (MA10) confirmed that MHC I elevation was fully shut down within the contaminated lung epithelial cells not like the lung epithelial cells contaminated with influenza virus. “So, here we found an intrinsically potent ability of SARS-CoV-2 to shut down the host MHC-I system,” Dr. Iwasaki tweeted.
Evasion technique
“We demonstrated that the ability to reduce MHC I expression remained unchanged throughout the pre-Omicron variant-of-concern evolution. These findings suggested three important perspectives on the MHC I evasion strategy of SARS-CoV-2,” they write.
First, the virus utilises a number of redundant strategies to suppress MHC-I expression. Second, MHC I downregulation could not solely impair cytotoxic T lymphocytes (CTL) recognition of contaminated cells for killing however may impair priming of T cells.
“Third, given that the variant of concern had not further evolved to down-regulate MHC I more strongly than the original strain except for the Omicron subvariants, the SARS-CoV-2 ancestral strain was already fully equipped to escape from T cell-mediated immunity with respect to downregulation of MHC-I expression and is under less evolutionary pressure to further optimize the evasion strategy,” they write.
“Our study provided evidence of inhibition of MHC I upregulation in SARS-CoV-2-infected cells in both in vitro and in vivo settings,” they observe.
“The cellular mechanisms and consequences of enhanced MHC I inhibition by Omicron variants on infection and disease remain to be determined,” they write.

